We will start off by considering that the spreading out of particles takes the most time to occur in a solid!
And that spreading out occurs the fastest in a gas!
Hi! 🙂
Diffusion is really about one thing actually…
which is… that
particles move about randomly!
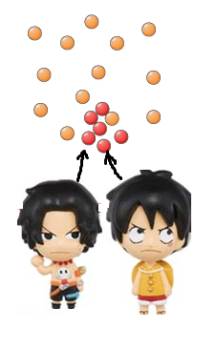 To give you an example… you and your friend walked into a party
To give you an example… you and your friend walked into a party
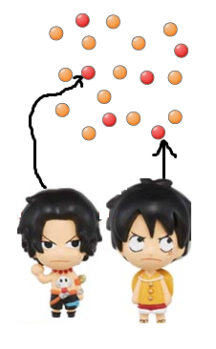 now, if both of you moved randomly, after a while, you will definitely spread out!
now, if both of you moved randomly, after a while, you will definitely spread out!
Spreading out takes time! But start out with particles next to each other, they will be further apart after a while by random movement, and eventually fully spaced apart!
Here is another example
Now think about the following experiment
you see the brown colour of bromine gas spreading to the top after the air in the two cylinders are allowed to mix!
Do you know why the colour of bromine becomes lighter after it has spreaded out?
The reason is because bromine has become LESS CONCENTRATED! 🙂
Next we will look at another example…
which is the very striking purple compound called Potassium Permanganate
Put crystals of Potassium Permanganate at the base of a conical flask and add water… you will see
The reason why this has happened is because
Again, take note that the concentration of Potassium Permanganate particles actually decreased as it spreads out into the liquid! 🙂
Hi I am freshly squeezed from a lemon! I am lemonade
Do I
taste sour? Yes!
Conduct electricity? Yes!
react with metals, bases, and carbonates? Yes!
The baking soda (a base) and the lemon juice (an acid) combine to release Carbon Dioxide gas. The liquid soap turns the bubbles into a foam that often erupts right out of the glass.
So I am lemon juice, and I am ACIDIC!
An acid can do all 3 things mentioned above!
An acid has pH less than 7!
Rain, wine et-cetera et-cetera all contain different acids! Rain’s acidity comes from dissolved carbon dioxide, while wine’s acidity comes from fermented grapes!
They all have pH below 7, so what is pH?
pH measures the concentration of hydrogen ions in something!
What are hydrogen ions?
First let us see what all acids have in common!
They all have H in them!
When in water, the H splits apart from the acid to become H+ ions! They left their electrons behind with their acid pal to form a negatively charged ion.
Why do you need water to be present to have H+ ions?
Because water has the ability to attract the H from the acid to split from the acid!
the hydronium (H3O+) here is the same as H+!
H+ ions are the big deal that makes acids who they are!
If you are an acid, you will react with metals, and other stuff… and that is all because your H+ ions are attacking those guys!!!
H+ makes an acid incredibly powerful
A really powerful acid releases all the H immediately to form lots and lots of H+ ions when it dissolves in water.
Whereas a weak acid does not release all of its H to form H+ ions, it may hold on to its H! 🙂
Compare the 2 pictures above, can you see that there are more H+ ions per unit volume for strong acids compared to weak acids?
This means that strong acids have a higher concentration of H+ ions than weak acids.
Recall that pH measures amount of H+?
Can you see that a strong acid contains a really large amount of H+ ions?
This explains why chemists need to take precautions when working with strong acids!
source:
Osmosis is the movement of water molecules from a region of higher concentration to lower concentration… (not forgetting the partially permeable membrane) But what on earth does it means? 🙂
Isn’t water just another compound? Why is it so special that there is a term called osmosis to describe how it moves?
It is actually quite simple to understand and you will find out that water is not really that special after all!
1st thing to note:
Large quantities of water molecules constantly move across membranes by random movement!
For example, it has been estimated that an amount of water equivalent to roughly 250 times the volume of the cell diffuses across the red blood cell membrane every second; the cell doesn’t lose or gain water because equal amounts go in and out.
2nd thing to take note of
Water molecules can dissolve other substances.
 You put a cube of salt into a cup of water and the cube of salt rapidly disappears!
You put a cube of salt into a cup of water and the cube of salt rapidly disappears!
The reason is because the water molecules attract the salt particles out of the cube…
and distribute the salt particles all around the liquid
That is why light can pass through the gaps and we cannot see our salt particles anymore!
3rd thing to take note of
Water molecules are locked with their solute particles because of the attraction towards the solute particles.
Take note of the cluster of water molecules that are binded to the solute! The other water molecules are free!
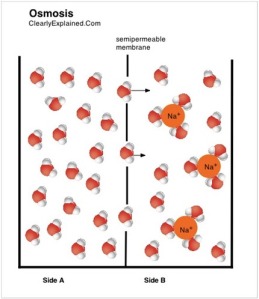
Take note of the water molecules that are also binded to the sodium ions! Take note of there is a lot more FREE water molecules on the left than on the right
4th thing to take note:
You must have a partially permeable membrane to block solute particles from going over to the other side
Now finally,
we can go into the idea that it is the free water molecules moving randomly that causes this phenomenon called osmosis
Notice that the volume on both sides of the membrane is the same
There is a lot more FREE water molecules on the right
So there is a higher concentration of FREE water molecules on the right compared to the left
Therefore by random movement alone, more water molecules will find themselves on the left side!
Same thing for this picture!
Last but not least, see if you understand this picture below
You will definitely encounter pictures of some visking tubing etc having higher water levels on one side than the other…
the reason is because osmosis balances out the number of FREE water molecules on both sides. But one side has more solute particles and binded water molecules to them so the water level is higher!
Hope you have gained a better understanding of osmosis! 🙂
source:
http://arbl.cvmbs.colostate.edu/hbooks/cmb/cells/pmemb/osmosis.html
http://serendip.brynmawr.edu/sci_edu/waldron/pdf/OsmosisProtocol.pdf
A reducing sugar is any sugar that has an aldehyde group, or can form one.
key thing 1
– is that an aldehyde group is needed, which must be present on either the 1st or the last carbon.
For sugars like maltose, glucose or lactose to be reducing sugars when they don’t appear to have an aldehyde group, the next point to note is
key thing 2
– the ring structure can be broken to reform an aldehyde on the 1st carbon just like the reversible reaction shown here for glucose.
After that, look closely at Maltose and Lactose to examine if the 1st carbon atom can split with the oxygen in the ring structure to form back an aldehyde on itself just as glucose could in the picture above.
For Maltose, the 1st carbon for the glucose residue on the right is free to reform into an aldehyde! Check that it is the same for Lactose below.
And last but not least, examine this picture of sucrose
Note that to form an aldehyde, the OH on the 1st carbon must give its H to the O in the ring… but there is no OH group on any of the 1st carbons in sucrose!
Aha!
The example of enantiomers that I will give here is alpha and beta glucose!
Alpha and beta glucose can’t be more similar in terms of structure, yet one forms the indigestible and tough cellulose while the other forms starch and other yummy goodies like lactose / sucrose.
as you can see from the middle structure, beta and alpha glucose are the one and the same.
The only difference as A level Biology tell us is that the OH on the 1st carbon is above the ring structure for beta glucose but the other way around for alpha glucose.
How to visualise it in 3d? take a look at the image below
can you spot which is the 1st carbon and which is the 4 carbon atom?
Did not you notice whether the OH is above or below?
Hi! We are looking at non-reflective coatings! These guys admit more light into cameras and other optical instruments.
The blue-violet colour produced by a non-reflective coating
Explanation of the appearance:
On axis, the lens looks dark – which means that little light is being reflected, so most is being admitted into the camera – which in turn is the chief purpose of non-reflective coatings: they make the instrument more sensitive to light.
How does the coating create such an effect?
The key idea is destructive interference
Destructive interference refers to waves canceling one another out.
If there is no coating, then light will only be reflected at the boundary between
– lens and air
If there is coating, light will be reflected at the boundary between
– air and coating,
– coating and lens. (see picture below)
The purpose of the coating is to provide another layer for incoming light to be reflected so that the reflected waves will cancel each other out.
see video: http://www.youtube.com/watch?v=1bGx0t50Gu0
if little energy is reflected due to destructive interference, then most of the energy must be transmitted into your eyes, or into the your camera!
Why do lenses appear to have a blue-green tinge?
The key thing to understand is the angle of incidence.
When the angle of refraction in the coating is θ, the pathlength difference is λ/(2n cos θ): longer by a factor of 1/cos θ. So the destructive interference is more complete for longer wavelengths – towards the red end of the spectrum – and the destructive interference is less complete for blue and violet. This explains why the lens in the photo above appears to havea blue-violet tinge – which provides a simple way of recognising such coatings.
These photograhs show the effects of non-reflective coatings on spectacles. In the photo at left, we see less light reflected from the spectacles with the coating (and, as explained above, the destructive interference is more effective at the red end of the spectrum, because the photo is taken off axis).
Practical benefits of having coating on your spectacles
The non-reflective coating allows more light into the spectacles – the eye receives more light. Another effect is that another observer can see the wearer’s eyes better, because less reflected light from the spectacles is added to that reflected from the wearer’s eyes.
source:
http://www.animations.physics.unsw.edu.au/jw/light/non-reflective-coatings.html
As it is, I was looking through a pdf document on ideas for caregivers of people suffering from Dementia. Some ideas include the following
– involvement in household chores
– casual walks
– simple ball games
– simple exercises
Hmm so much so for the exercises, but I was thinking that one caregiver can only do so much…
What if dementia patients come together, support each other, and play games with each other?
This would free up a lot of time and energy for caregivers, assuming they were individually taking care of individual dementia patients.
I do think that with the rise of social media and stuff nowadays, it may just be likely that one day the elderly, special needs people, chronic illness patients of the community can come together in a public space, look after each other, and interact with caregivers from across the community.
Hmm, as a buyer of fruit juice containers, occasionally I wonder if these containers which are made of tetra-pack can be dropped into the plastic recycling bin for recycling…
So I happen to drink Marigold “peel fresh” brand of juice and the bottle does not contain any recycling sort of mark or anything. So I did some research and I read somewhere that tetra pack containers indeed can be deposited in the “plastic” recycling bin and it will be sorted out at the recycling centre.

Hmm, I start to wonder if I can write in to the company and suggest that they incorporate some sort of recycling mark so that fellow buyers of such juice will know and be reminded that these containers can be recycled.
But of course I have zero faith that the company will adopt my suggestion anyway. I kind of wonder if I have to build a business case to justify why they should add the mark or some description… like perhaps find out how many containers were actually recycled against the numbers that were manufactured or sold.
To do so, one interesting method I could do is to do a poll of people to ask them if they know that tetra-pack containers can be recycled. If a lot of people responded “no” then it gives some justification.
This interestingly makes me a social activist already!
Hmm defined by a website from Amherst College as
Social activism is an intentional action with the goal of bringing about social change. If you feel strongly about a cause and are working towards a change, you could be considered an activist.
I am interestingly being one. So I first got a flavour of social activist from listening to audio tapes on the history of public health using my iphone using this app called itunes u. In summary, a lot of individuals have stood up and made a difference in getting governments to recognise that they need to tackle public health which shows that you know, you can make a difference too!
You can look at the very good public health audio tapes from John Hopkins by following this link:
https://itunes.apple.com/us/course/the-history-of-public-health/id535929070
Now Newton’s 3rd law is one of the most un-intuitive stuff out there. It states that every force has an equal and opposite reaction. You push something and it pushes you back with the same force.
Let examine it from the perspective of an aeroplane

You have here an aircraft engine. So air is being sucked into the engine and so it moves from left to right. The movement of the propeller creates a FORCE that is a PULL which sucks air into the propeller.
You can see the opposite force it creates here

Meanwhile, a helicopter works in the same way. the wind is sucked downwards by a pulling downwards force created by the rotation of the helicopter blades. So the helicopter moves up…. and that is why when a helicopter is overhead you can feel super strong gushes of wind sweeping you.
Alright, the most cited example of the law is that of simply being able to walk because you exert a force on the floor in the opposite direction you want to go, and can feel the floor’s reaction force on you. Try it using the image below!

For something totally un-related, try learning the basics of flight using the following links that I found while searching for the pictures of aeroplanes…